The nuclear pore complex (NPC) is a shrewd gatekeeper. Dotting the surface of the nucleus, these protein complexes comprise the sole portals in and out of the cell’s command center, granting passage only to select molecules. NPCs are essential to the cell’s survival, and even minute abnormalities in their components may contribute to a host of diseases, from heart conditions to cancer to Alzheimer’s.
Now, a new study published in Cell provides a comprehensive, three-dimensional structure of the nuclear pore complexes of yeast cells. “This is a big breakthrough for us,” says Rockefeller’s Michael P. Rout. “Our model reveals novel features of the various parts of the pore’s structure, giving us new insights into how this sophisticated gateway evolved, maintains its architecture, and functions as a transport machine. A better understanding of the basic biology of the NPC will hopefully lead to new insights in its role in human disease.”
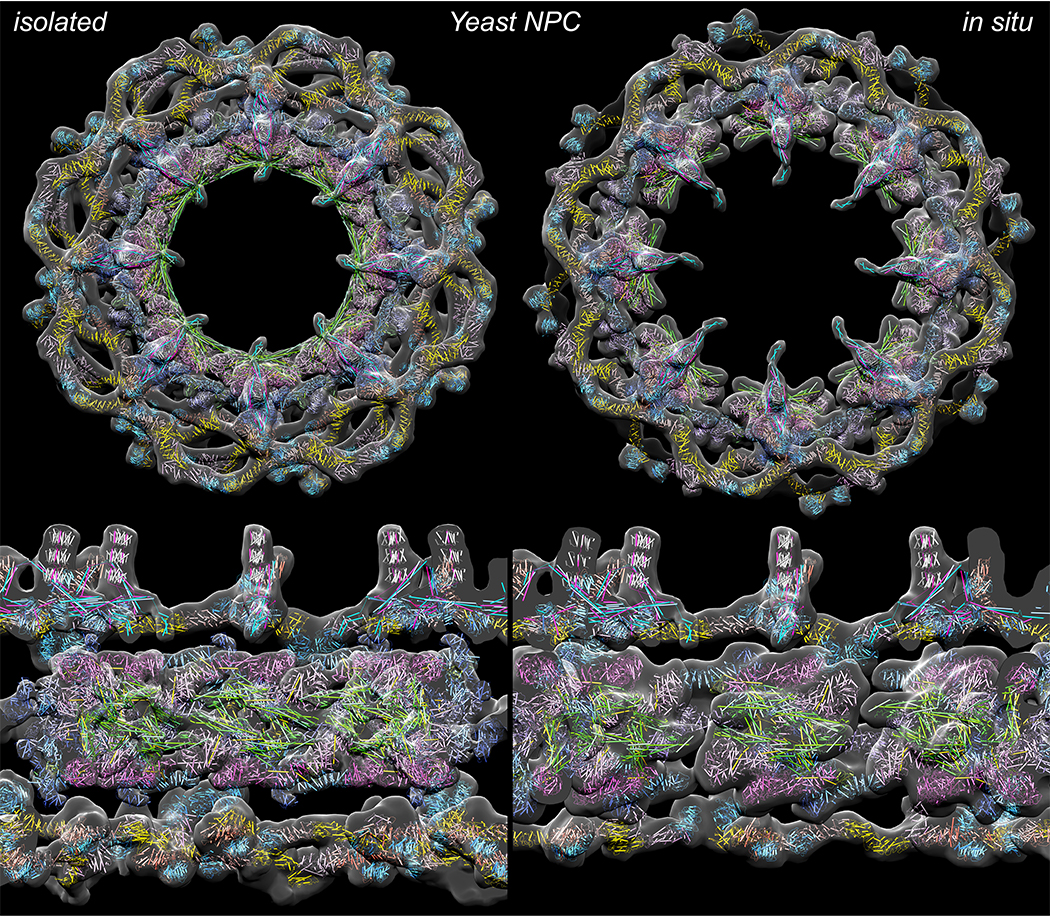
They identified 28 flexible connectors that tie the inner ring of the complex together and likely aid in rapid assembly and disassembly of the NPC during cell division. Crucially they also addressed a question that has stumped the field for many decades, namely: are all NPCs in a single nucleus essentially the same? They have found that the answer is a definitive “no”: at least three very distinct variants can coexist within a single yeast cell, each potentially specializing in different functions such as the transport of different proteins or RNA, say Rout and Chait.
The findings are the latest in a decades-old quest to understand how molecules enter and exit the cell nucleus, and follow from a 2018 study in which Rout and Chait identified the 552 nucleoporins of the yeast pore complex and determined how they fit together.
Access the full text here!
