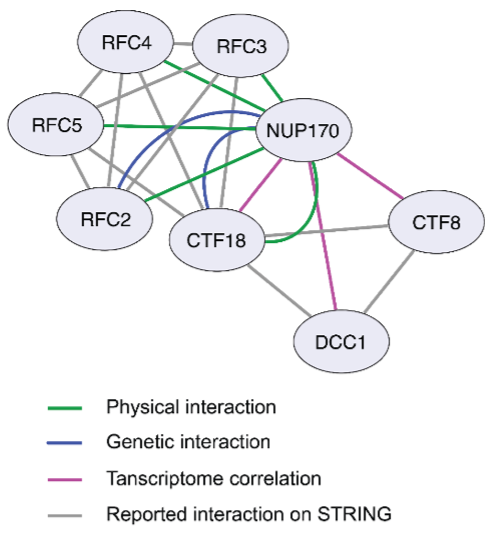
Figure 1: Cytoscape network illustrating the interaction between Nup170 and the Ctf18-RFC complex.
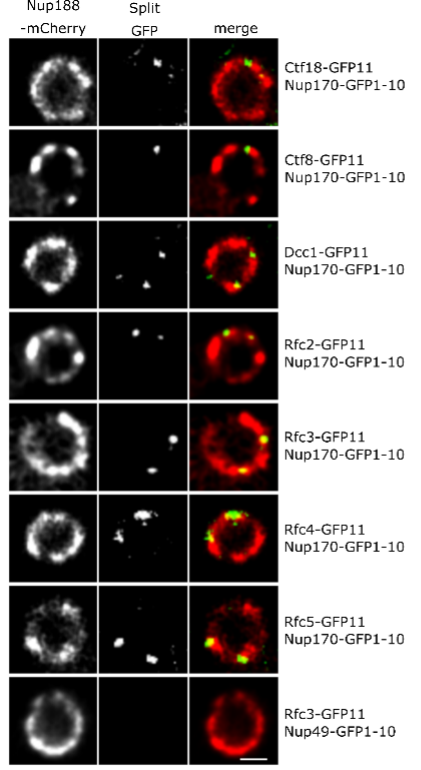
Figure 2: Ctf18-RFC is recruited to a subset of NPCs lacking the nuclear basket nucleoporins Mlp1 and Mlp2.
The Nuclear Pore Complex (NPC) functions as a crucial gateway, mediating the transport of macromolecules between the nucleus and the cytoplasm, thus playing a central role in regulating gene expression. In addition, the NPC’s physical interaction with chromatin significantly affects transcription, chromatin structure, and DNA repair. For example, the interaction of active genes with the NPC propels transcription, while interaction with telomeres fosters transcriptional silencing. The intricacies of these interactions, requiring different core channel proteins like Nup170, underscore the multi-faceted roles of NPCs in cellular functions. Mutations in NPC components can be deleterious, and potentially linked to a myriad of diseases, thereby amplifying the importance of in-depth structure-function studies of the NPC.
Our recent work, published in the Journal of Cell Biology (JCB), utilized an integrative systems cell biology approach encompassing genomic, proteomic, transcriptomic, and biochemical methods to provide mechanistic insight into how Nup170 facilitates its regulatory role over gene silencing. As part of our analysis, we compared the transcriptome signature of cells lacking Nup170 to a compendium known as ‘the deleteome’, which contains the transcriptomic profiles of approximately 1,500 individual yeast gene deletion strains. We pinpointed the deletion strain of Replication Factor C (RFC) complex member Chromosome Transmission Fidelity 18 (ctf18Δ) as having a similar transcriptomic signature as the nup170Δ strain (Figure1). Additionally, RFC complex members physically associate with Nup170, as identified by affinity purification mass spectrometry methods.
What is the significance of these observations? To our surprise, we found that Nup170 has a role in the Replication Factor C (RFC) complex-mediated loading of Proliferating Cell Nuclear Antigen (PCNA) onto DNA. PCNA serves as a sliding clamp to enhance the processivity of DNA polymerase during DNA replication and repair, but it also acts as a versatile hub in a myriad of additional cellular processes, including DNA mismatch repair, chromatin remodeling, and cell cycle regulation. Notably, upon Nup170 loss, a genome-wide decrease in PCNA levels was observed, as opposed to a telomere-confined decrease, suggesting a broader, systemic role of the NPC in mediating PCNA loading functionality. Furthermore, our experiments revealed that this functionality of Nup170 is specific to a subset of NPCs that lack a nuclear basket (Figure 2). To determine if PCNA levels are a critical factor in controlling telomeric silencing, we combined the nup170Δ with a PCNA unloading factor mutant (elg1Δ). Indeed, combining the loss of Nup170 with the loss of Elg1 resulted in increased PCNA levels and we also observed normal transcriptional silencing of sub-telomeric genes. Thus, increased PCNA levels on chromatin by the inactivation of an unloading factor is sufficient in maintaining telomeric silencing. These findings represent a leap forward in understanding the functional specialization of NPCs, specifically, the role of Nup170 in DNA damage repair and heterochromatin formation (Figure 3).
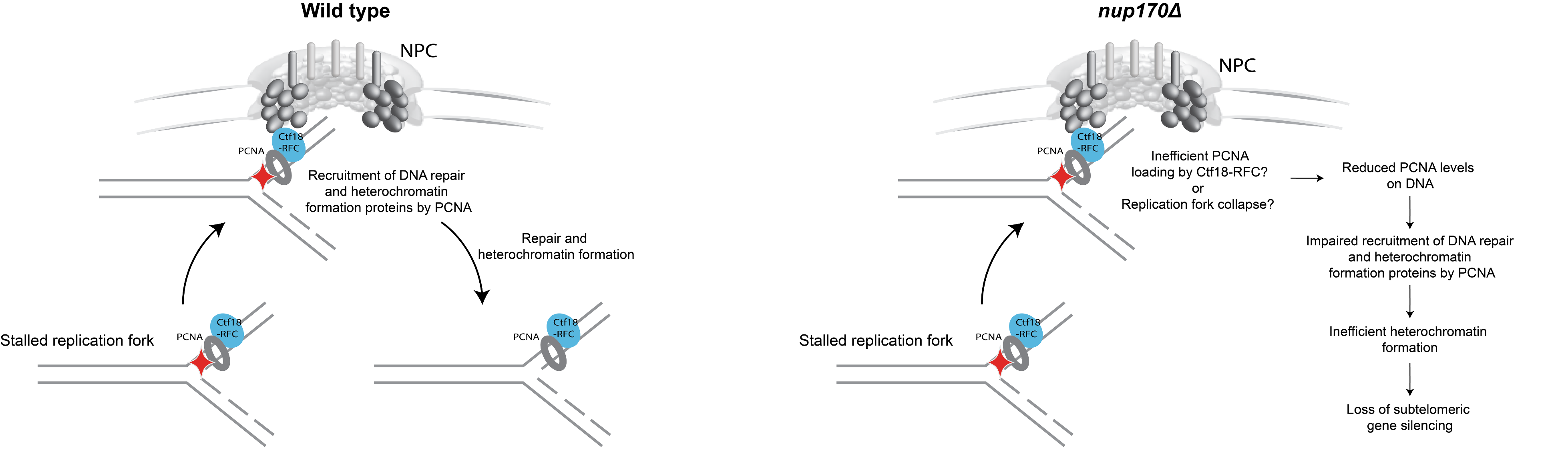
Figure 3: Model illustrating the role of the NPC in DNA damage repair and heterochromatin formation.
Publication:
Kumar S, Neal ML, Li S, Navare AT, Van Eeuwen T, Wozniak RW, Mast FD, Rout MP, Aitchison JD. “Nuclear pore complexes mediate subtelomeric gene silencing by regulating PCNA levels on chromatin.” J Cell Biol. 2023 Sep 4;222(9):e202207060. doi: 10.1083/jcb.202207060. Epub 2023 Jun 26. PMC10292210
The paper can be freely accessed here:

