NCDIR scientists at The Rockefeller University: Dr. Dom Olinares (left) and Prof. Brian Chait, assisted fellow Rockefeller scientists in the lab of Dr. Jue Chen by using Native mass spectrometry to aid structural biology in determining the structure of a substrate-bound peptidase-containing transporter!
Peptidase-containing ATP-binding cassette transporters (PCATs) are a unique class of transmembrane transporters that secrete antimicrobial or quorum-sensing peptides. PCATs contain peptidase domains fused to the transmembrane transporter segments that cleave the polypeptide substrate prior to translocation.
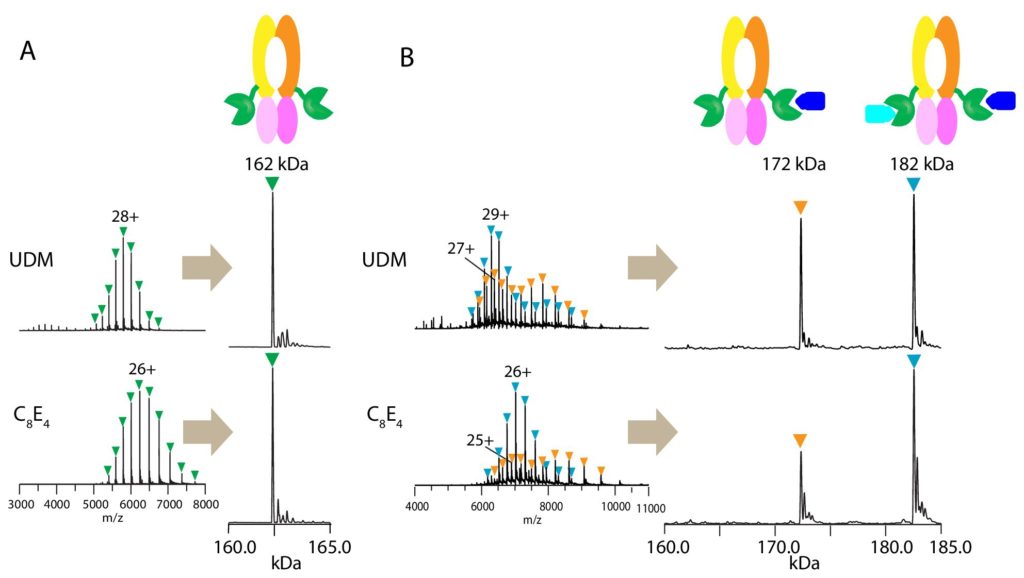
Native mass spectrometry (MS) enables direct mass measurement of intact protein assemblies providing critical information on the composition and stoichiometry of protein complexes for integrative structural biology studies. NCDIR scientist Dom Olinares from the Chait Lab used native MS to determine the assembly state of and the number of bound substrates in the PCAT1 complex. The resulting native MS information greatly helped in the structural analysis of the transporter using cryo-electron microscopy by the laboratory of Jue Chen at The Rockefeller University.
Native MS analysis revealed that up to two substrates can bind the homodimeric PCAT1. The high resolution, cryo-EM structure of PCAT1 in complex with its substrate showed that two substrates are bound to the transporter, yet only one is positioned for cleavage and translocation. Overall, the study yielded insights on how substrate cleavage, ATP hydrolysis, and substrate translocation are coordinated in a PCAT transport cycle.
For more information, you can access the paper here: https://elifesciences.org/articles/51492